Kieran Gallagher
Scientific
Explorations
In Fall 2022, I started a Chemistry Ph.D. Program at Portland State University, where I work in The Regional Laboratory for the Science of Cultural Heritage Conservation. Below you can find projects I have been a part of, in conjunction with museums, librarys, and cultural centers in the Pacific Northwest.
The Chachalu Museum, located in Grand Ronde, Oregon, loaned us a few objects to study- three glass containers that have been woven around and one woven pipe. We studied these objects using XRF, 2D and 3D x-ray imaging, and DART-MS and were able to find tidbits of information about the objects.
I was lucky enough to present this information at the 2023 Grand Ronde History and Culture Summit.
Woven Pipe
The museum noted that they thought there was metal inside the pipe, which was confirmed through 2D X-Ray imaging. I was not expecting the metal to run through the whole pipe as we saw, but it makes sense, as it was probably used as a support for weaving the pipe.
If you look closely in both images, you can see the metal protruding from the bowl of the pipe. We were able to XRF on this exposed bit to see the atomic makeup of the metal and found that it was mostly iron, which suggests it is a steel of some sort.


An X-Ray image of the pipe. The darker line is the metal running the length of the pipe.
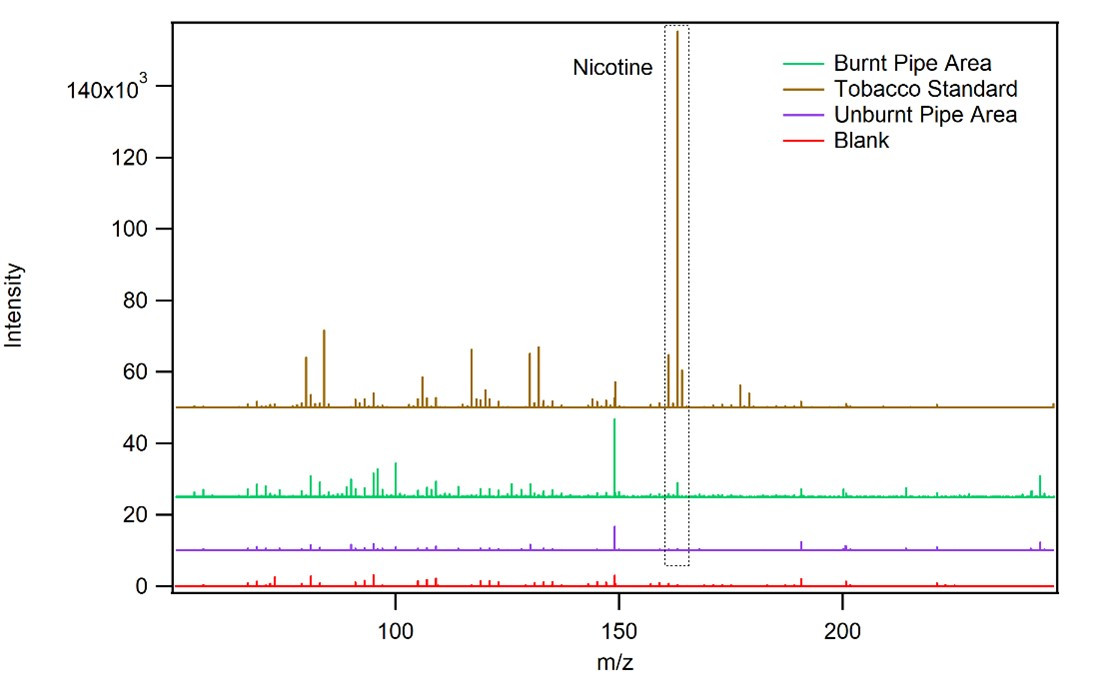
The bowl and heel of the pipe are slightly burnt, leading to questions about if someone tried to smoke from this woven pipe. Via Direct Analysis in Real Time (DART)-MS, we were able to detect nicotine and anatabine residues on the burnt area, suggesting that tobacco was burned in this pipe.
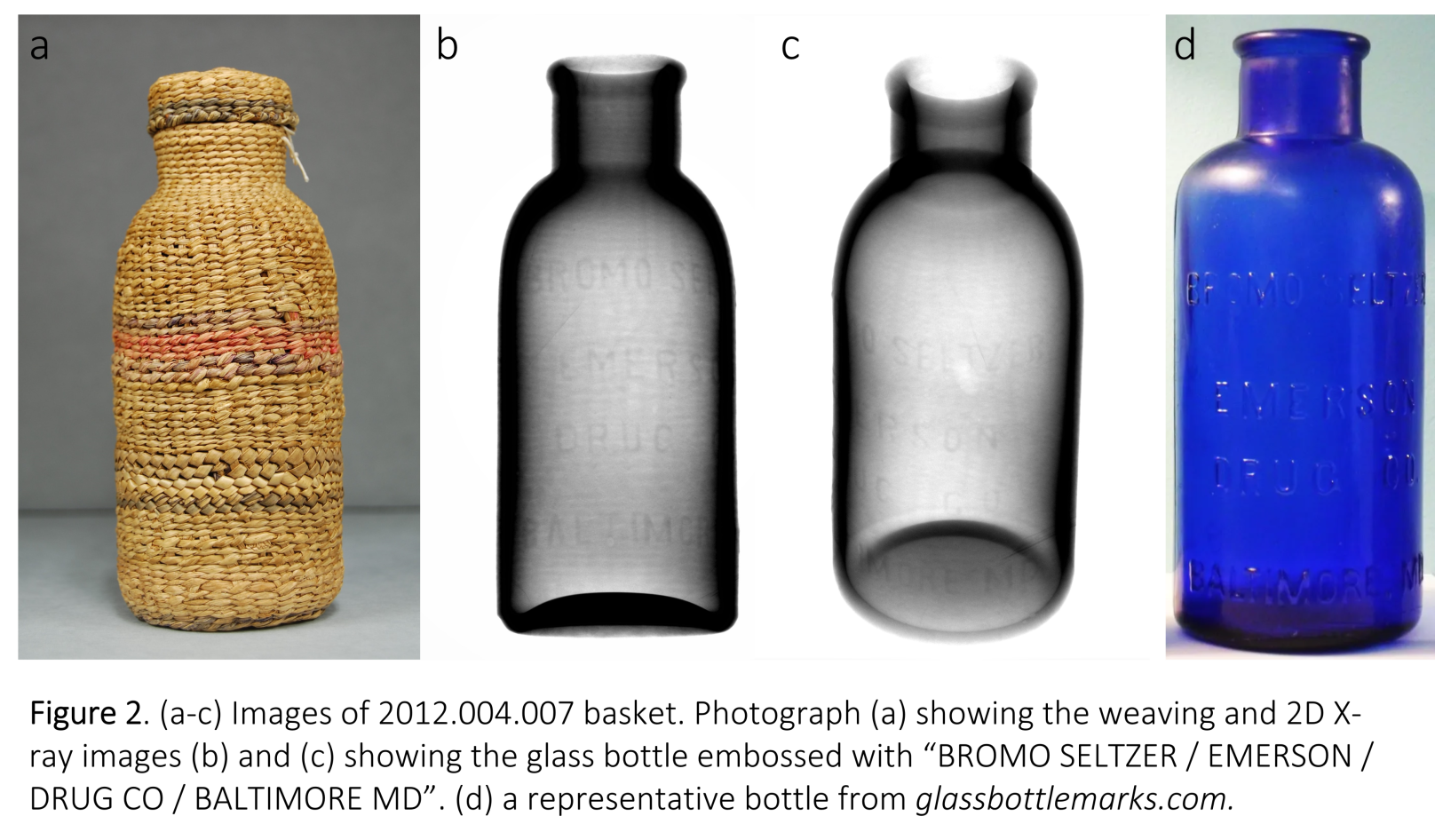
Labeled Bottle
This smaller bottle, also woven, hid labeling on the glass that could be seen through X-Ray Imaging. The label (written below) placed the creation of the bottle to around the 1920's, meaning that the basketry element had to be woven after that. The cap is an interesting feature, and not seen super often with objects like these.
Threaded Bottle
When this basket was analyzed through CT reconstruction, a lovely treat was revealed- the glass had a ring of trees embossed into it! This was a really cool thing to find, and we have not been able to find anything like it to help us date it or learn more information about its production. However due to the fact that it is threaded and has a seam, it was most like produced post-1930.

Throughout most of the early 1800's, arsenic was a key ingredient in two green pigments: Scheele's Green and Emerald Green. Scheele's Green was commonly used in wallpaper and paints, and even some childrens toys. Emerald Green was used as a paint and a fabric colorant, but also as an insecticide (and yet thought it was fine for humans...?). Of course, arsenic is toxic to humans in very low concentrations, so these pigments were phased out by the late 19th century.
These pigments were also used in book bindings, and more likely than not, on a book in a library near you (panic! well maybe not that but). So The University of Washington Library had some books (50) that they wanted to test for arsenic. In total, we found that 9 of them contained arsenic using X-Ray Fluoresence. Raman spectroscopy was used to determine that all books were pigmented with Emerald Green.

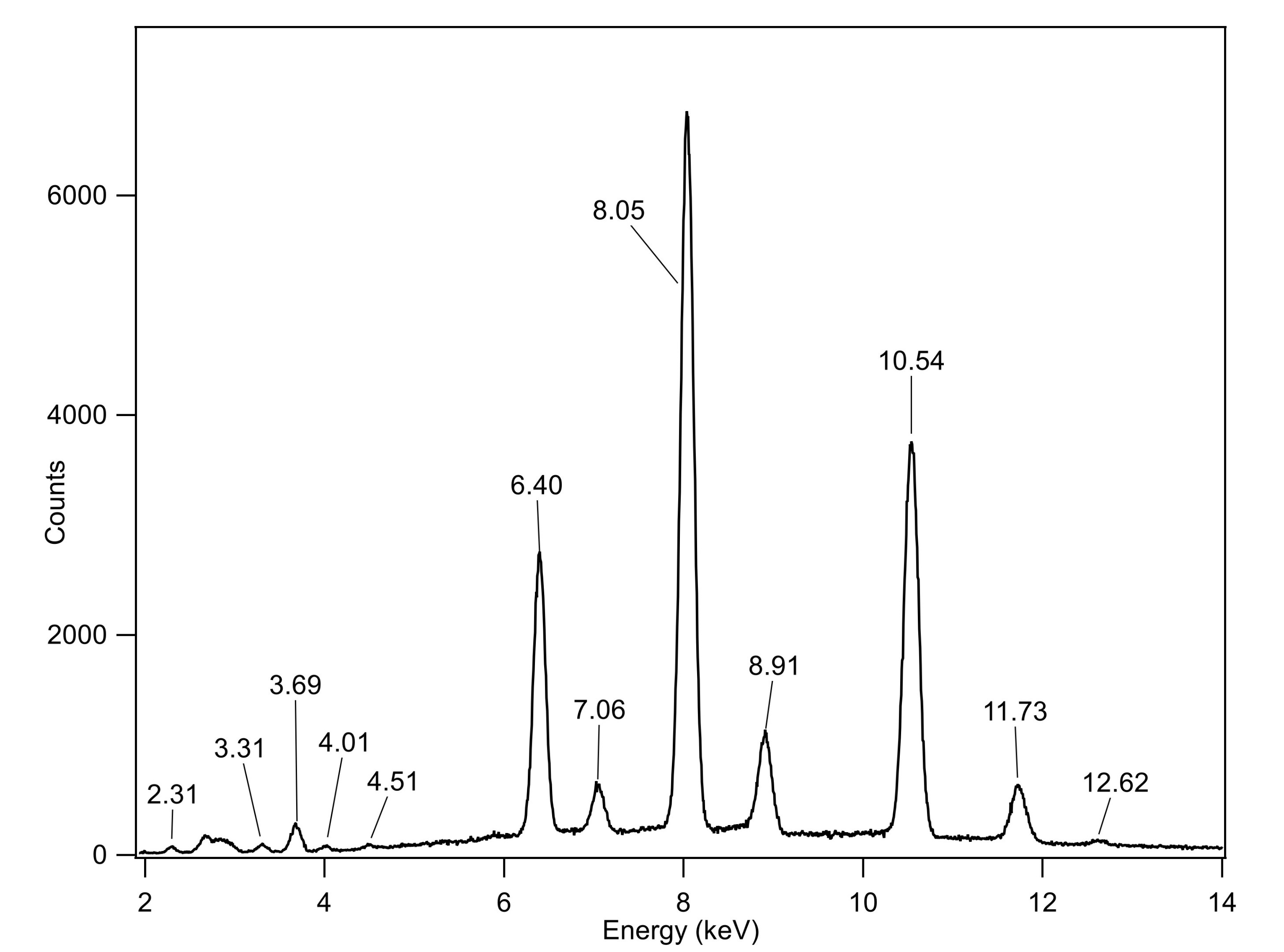
If you are wondering if a book you have uses an arsenic green, they were usually produced between 1830 and 1870, and are highly decorated with gold titling or detailing. The arsenic green looks like the book shown below (along with its XRF spectrum). To learn more, check out the Poison Book Project.
Copper Corrosion Detection
Corrosion is a serious concern for those tasked with maintaining metal sculptures and structures. Slowing the process of metal corrosion will save money and prolong the lifetime of culturally significant artworks by reducing the need for corrosion removal and metal replacement. Slowing corrosion can also mitigate climate change, as replacing corroded metal could account for almost 10% of total CO2 emissions by 2030. Corrosion can be slowed with the application of protective films, such as coatings or inhibitors. Monitoring metalworks for early corrosion signs can indicate failures in protective films which will allow them to be replaced, minimizing further corrosion.
While there are methods of detecting corrosion on copper-based metals, they do not easily differentiate between Cu(I) and Cu(II) ions due to the disproportionation of Cu(I) in aqueous solutions. Determining the severity of copper corrosion is challenging because cuprite (Cu2O) is a common, often desired patina that does not always indicate active corrosion. The following work will produce a fast and effective way to detect corrosion, and assess severity by identifying Cu(I) and Cu(II) separately through colorimetric and electrochemical analyses via hydrogels. Conservators will be able to use the produced hydrogels to determine the best course of action to preserve copper-based artworks.
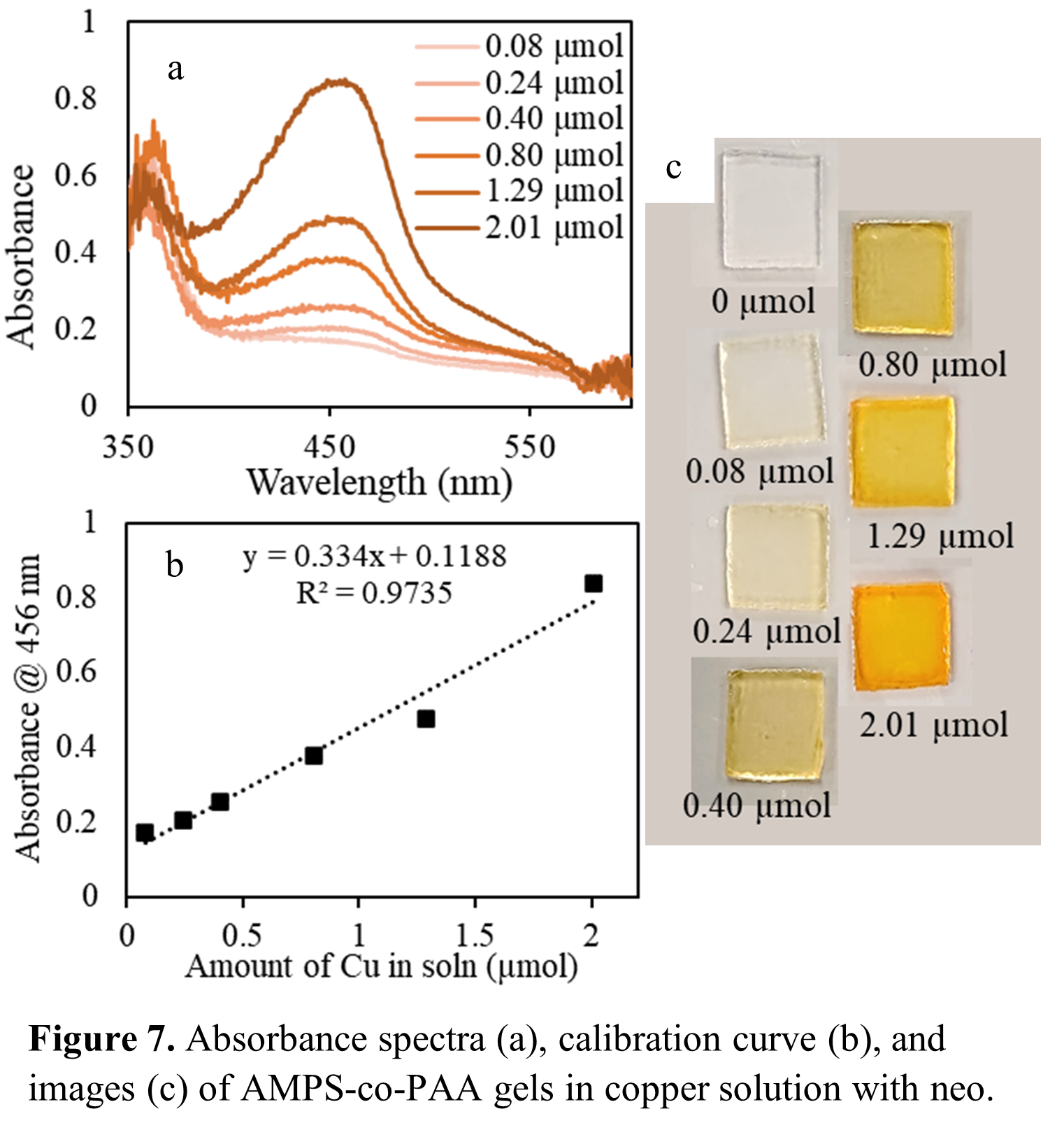
Using a modified hydrogel produced in our lab, I have shown that Cu(I) concentrations can be quanitified photometrically through complexation with neocuproine. This can be applied to determine how effective coatings are, by detecting mass transport and defects, as well as determine the oxidation level of copper corrosion along with other methods shown to detect Cu(II), like HCF(III).
This method is selective: no other ions interfere with the Cu(I)-neo interaction.
Along with this work, I am synthesizing new hydrogels that uptake copper ions more efficiently, as well as synthesizing neocuproine derivatives that polymerize into the hydrogel and still colorimetrically indicate the presence of copper. This work will be tested on copper test plates to confirm its ability to detect coating defects, and current work on the subject is promising.
Materials Characterization for Cultural Heritage
In Spring of 2025, I was lucky to be a teaching assistant on a spring break trip to Greece, with the above title. The course visited a number of incredible museums such as the Acropolis Museum, the Archaeological Museum of Thebes, and the Archaeological Museum of Delphi. We were also able to tour the conservation facilities at Byzantine & Christian Museum in Athens, as well as get a special pass to see the Erechtheion up close on the Acropolis and talk with conservators on site.

This trip was in partnership with the University of West Attica's Conservation program, so we recieved lectures from convervation professors Yorgos Facorellis, Vasilike Argyropoulos, and more. After the trip, Dr. Clare (my PI) and I had a meeting with Dr. Argyropoulos and Dr. Stamatis Boyatzis to possibly facilitate future collaborations.
I had an absolue blast on this trip, learning more about conservation and the different ways that scientists can support their work.